Metalloisoporphyrins
J. Krumsieck, Ç. Baş, J. Rusche, C. Knaus, W.-D. Möller, N. Schulze, D. Körner, M. Bröring Poster
ICPP-12, 10.07.2022 - 15.07.2022 in Madrid.
KCT, 07.09.2022 - 09.09.2022 in Jena.

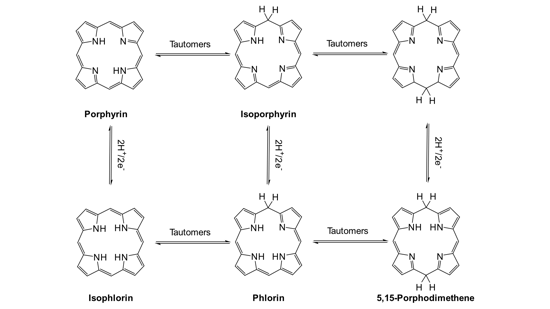
Isoporphyrin is a tautomer of porphyrin with broken aromaticity due to the presence of a meso-sp3 -carbon. This monoanionic ligand structure is of interest because of the Q-bands in the NIR region of the optical spectra. Isoporphyrin structures are discussed in possible intermediates in chlorophyll synthesis and in the catabolism of heme.
Selected Publications:
- R. B. Woodward, Angew. Chem. 1960, 72, 651.
- J. Bhuyan, Dalton Trans. 2015, 44, 15742.
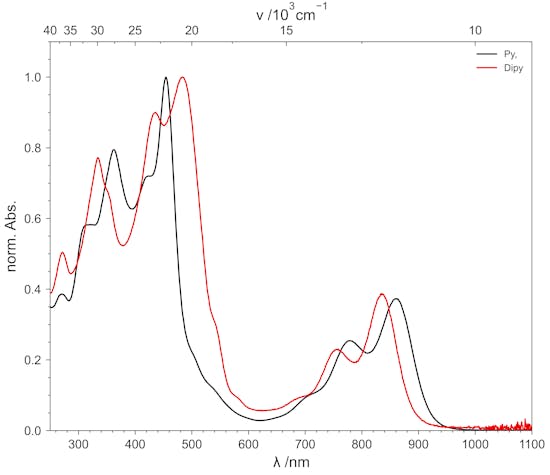
Zinc- & Cadmium-Isoporphyrins
Zinc- and cadmium complexes of pyrrolyl and dipyrrinyl isoporphyrins with different phenyl and thienyl groups in meso position are reported. Starting from pyrrol or a dipyrromethane those complexes can be prepared in a template mediated two-step-one-pot synthesis. Zinc or cadmium acetate can be used as template reagents.
The Procedures can be found in our publications in Angew. Chem. and Chem. Eur. J.
- Ç. Baş, J. Krumsieck, W.-D. Möller, D. Körner, M. Bröring, Chem. Eur. J., 2021, 27, 8021 – 8029, DOI:10.1002/chem.202100686.
- P. Schweyen, M. Hoffmann, J. Krumsieck, B. Wolfram, X. Xie, M. Bröring, Angew. Chem. Int. Ed., 2016, 55, 10118, DOI:10.1002/anie.201604297.
Besides H3-Corrole and Zinc-Porphyrin there a other interesting side products forming in this synthesis. For example a Zinc-5,15-Bispyrrolylporphodimethenes and Zinc-Dipyrrins and Zinc-Tripyrrins. A recent paper about these sideproducts has been published in Eur. J. Inorg. Chem.
- Ç. Baş, F. Doettinger, N. Klein, S. Tschierlei, M. Bröring, Eur. J. Inorg. Chem. 2021, 2021, 3239 DOI: 10.1002/ejic.202100472.
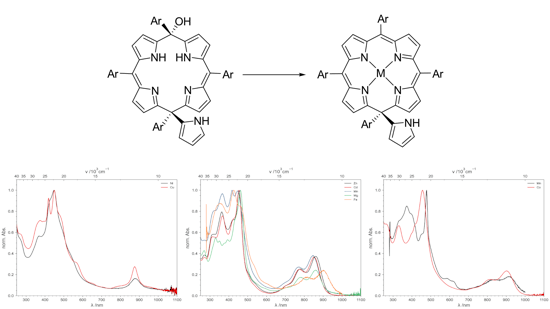
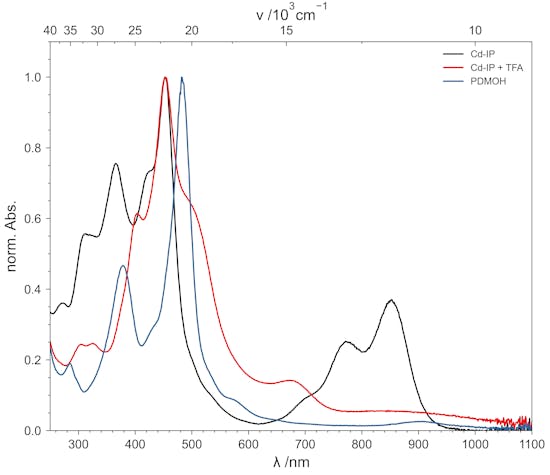
The Free Base is a Hydroxyporphodimethene
Treatment with strong acids is often described as decomposition of the ligand. This is not the case. Repeated washing with TFA and acetate buffer (pH = 2 to 4, 0.5 molar) followed by sodium carbonate (for electron donating meso substituents) leads to a metal free species which turned out to be a hydroxyporphodimethene.
Remetalation via Acetate Method
Remetalation of the Porphodimethene can be achieved by using the well-known Acetate Method. The metal-free species and the corresponding acetate salt are heated in glacial acetic acid. The transmetalated Isoporphyrin can be obtained with nearly quantitative yields.